Last Updated on June 11, 2017 by Editor Futurescope
Scientists at the Lawrence Berkeley Laboratory and the University of California have discovered that a metal possesses the property of conducting electricity without producing heat – it is undoubtedly a most important finding that already defies the laws that hitherto apply to drivers. This discovery can mark a before and after in the scientific-technical world.
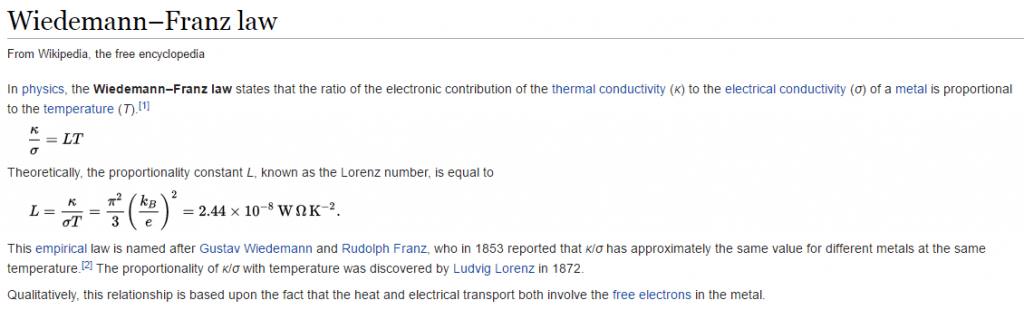
There is a conduction law, in physics, known as The Wiedeman-Franz law that establishes for metals that the ratio of thermal conductivity to electrical conductivity is proportional to the absolute temperature multiplied by the proportionality constant –Wikipedia. In simple words, it tells us that if a material conducts electricity, it also conducts heat.
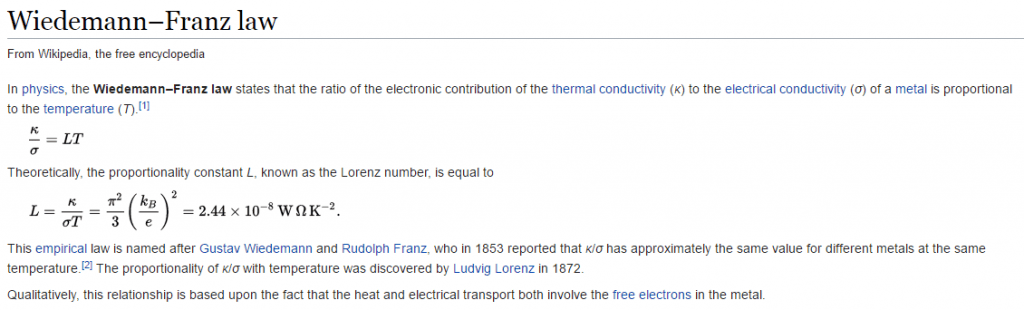
The metals are characterized by being good electrical and thermal conductors, generally any metal that conducts electricity suffers what is called the Joule effect, and this is due to the collision between the electrons that circulate through said material. This physical reality is regulated according to the Law of Wiedemann-Franz, which has hitherto served to explain the heating of metals by conducting electricity therethrough. Basically the good conductors of electricity will also be proportionately good heat conductors, so things like motors and appliances are heated when used regularly.
But a team in the United States has shown that this is not the case with metallic Vanadium Dioxide (VO2) – a material that is already well known for its strange ability to move from an insulator to a metal conductor at a temperature of 67 degrees Celsius (152 degrees Fahrenheit). “This was a totally unexpected find,” said lead researcher Junqiao Wu, from the Materials Science Division of the Berkeley Laboratory. “This shows a drastic error in textbooks that set out concepts about conventional drivers. This discovery is of fundamental importance for the understanding of the basic electronic behavior of the new drivers “.
This unexpected property not only changes what we know about drivers, it could also be incredibly useful – metal could one day be used to turn the lost heat from engines and appliances into electricity, or even create better window coverings that hold buildings Fresh. Many of handful other materials that conduct electricity better than heat that already known by many researcher. But only show those properties at temperatures of hundreds of degrees below zero, making them very impractical for any real-world application.
To discover this new and strange property, the team examined the way electrons move inside the crystalline network of vanadium dioxide, as well as the amount of heat generated. Unexpectedly, the team found the vanadium dioxide that causes the thermal conductivity attributable to the movement of electrons to be up to ten times lower than that dictated by Wiedemann-Franz’s law.
The reason for this seems to be the synchronized way in which the electrons move through the material. “The electrons were moving in unison with each other, much like a fluid, rather than individual particles as in normal metals,” Wu said. “For electrons, heat is a random motion, normal metals carry heat efficiently because there are so many different possible microscopic configurations that individual electrons can jump.” “On the contrary, the coordinated motion of the electron-band marching in vanadium dioxide is detrimental to heat transfer since there are fewer configurations available for the electrons to jump randomly,” he added.
By collaborating vanadium dioxide with other materials, researchers could “fine-tune” the amount of electricity and heat it could carry, which could be incredibly useful for future applications. It reveals that vanadium dioxide could help dissipate the heat of a system when they added metallic tungsten to vanadium dioxide that lowered the temperature at which the material became metallic, and also made it a better heat conductor. Only by conducting heat when it reaches a certain temperature. Before that, it would be an insulation. Unique properties of Vanadium dioxide can be transparent to about 30 degrees Celsius (86 degrees Fahrenheit), but then reflects the infrared light above 60 degrees Fahrenheit while remaining transparent to visible light.
The researchers at Berkeley have not discovered vanadium dioxide itself, but a property of that compound hitherto unknown, and is that the thermal conductivity attributable to the movement of electrons is ten times less than would dictate the law of Wiedemann-Franz. The physicist at the University of Berkeley and visible head of this discovery, Junqiao Wu, explains:
- In vanadium dioxide, the electrons move in unison, as in a fluid,
- Instead of in all directions as in the common metals.
- For electrons, heat is a random motion. Normal metals
- Transport the heat in such an efficient way because there are many possible
- Microscopic configurations by which electrons can travel.
- In vanadium dioxide, the coordinated motion of the electrons, as if they were
- The band of a parade acts to the detriment of the thermal conductivity.
- It is an effect of the smallest number of configurations available for the electrons

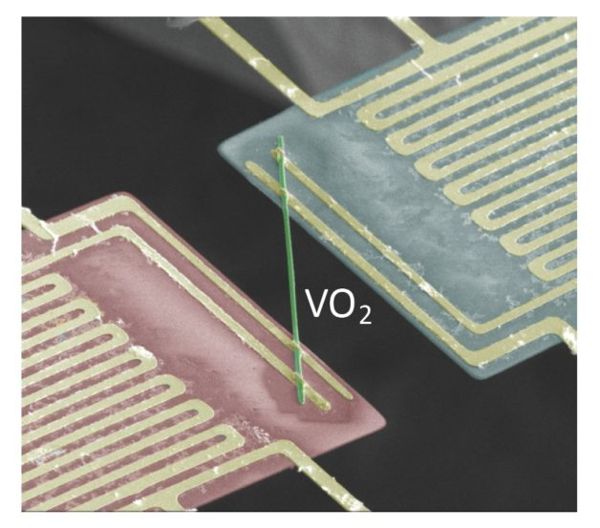
It is not the first metallic compound to transmit electricity better than heat. The difference is that it does so at room temperature rather than alone when subjected to impossibly low temperatures. The best part is that the amount of electricity it transmits can be adjusted by adding small concentrations of other metals. Vanadium dioxide has some other interesting properties. It is transparent to temperatures of 30 degrees Celsius, and absorbs infrared radiation above 60 degrees.
There are still some tests before marketing the compound, but its new feature has multiple applications. It can be used, for example, as a system to dissipate heat in motors while generating electrical energy. It would also make excellent heat insulation in windows.
So that means it could even be used as a window cladding that reduces the temperature without the need for air conditioning. “This material could be used to help stabilize the temperature,” said one of the researchers, Fan Yang. “By adjusting its thermal conductivity, the material can dissipate heat efficiently and automatically in the hot summer because it will have high thermal conductivity but will avoid heat loss in the cold winter due to its low thermal conductivity at lower temperatures ‘. Much more research on this disconcerting material needs to be done before it is further marketed, but it is quite exciting to know these strange properties exist in a material at room temperature.
It is certainly a material with great potential and you can find many utilities – such as reducing the heat in the phones. The applied use of this material can be revolutionary to develop electronic components, motors, etc. At the moment, the scientists are carrying out more tests to propose their exploitation at industrial level.
Read another Post on Metallic hydrogen is a reality on Earth: “the holy grail” of high pressure physics